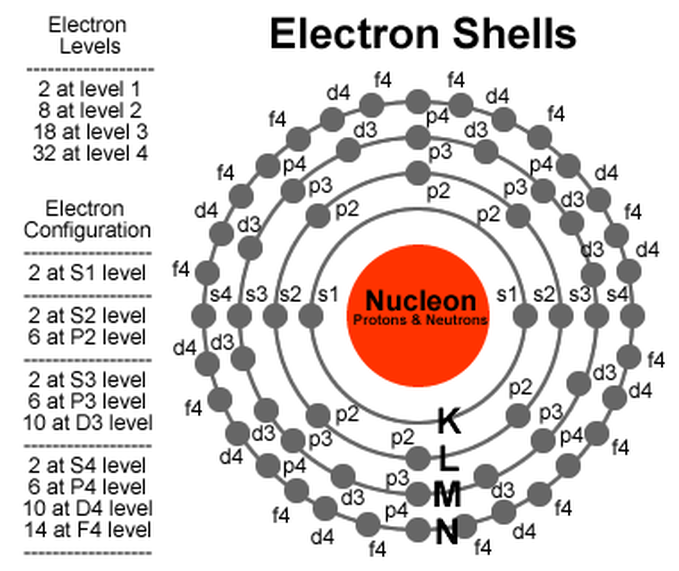
What Are Electron Shells Called . an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. The energy levels for the electrons in an atom are often referred to as electron shells. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. A full valence shell is the most stable electron.
from acemichael888.weebly.com
electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. A full valence shell is the most stable electron. energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. The energy levels for the electrons in an atom are often referred to as electron shells.
Electron Arrangement in Atoms Elements and the Periodic Table
What Are Electron Shells Called energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. A full valence shell is the most stable electron. energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The energy levels for the electrons in an atom are often referred to as electron shells. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons.
From www.slideserve.com
PPT Electron Energy Levels PowerPoint Presentation, free download What Are Electron Shells Called the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. The energy levels for the electrons in an atom are often referred to as electron shells. A full valence shell is the most stable electron. an electron shell, or main energy level, is the part of an atom where. What Are Electron Shells Called.
From www.nagwa.com
Lesson Video Electron Shells Nagwa What Are Electron Shells Called The energy levels for the electrons in an atom are often referred to as electron shells. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. energy. What Are Electron Shells Called.
From lessondbchandelles.z21.web.core.windows.net
Bohr's Atomic Model Notes What Are Electron Shells Called the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. The energy levels for the electrons in an atom are often referred to as electron shells. energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. an electron. What Are Electron Shells Called.
From www.youtube.com
Electron Shells and the Periodic Table IGCSE Chemistry YouTube What Are Electron Shells Called A full valence shell is the most stable electron. The energy levels for the electrons in an atom are often referred to as electron shells. energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. group 18 elements (helium, neon, and argon are shown in figure 2) have. What Are Electron Shells Called.
From www.britannica.com
Electron shell Definition & Facts Britannica What Are Electron Shells Called A full valence shell is the most stable electron. energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The energy levels for the electrons in an atom are. What Are Electron Shells Called.
From www.scienceabc.com
Octet Rule Definition, Explanation, Exceptions And Examples What Are Electron Shells Called group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting. What Are Electron Shells Called.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation ID83594 What Are Electron Shells Called the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The energy levels for the. What Are Electron Shells Called.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table What Are Electron Shells Called energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. A full valence shell is the most stable electron. group 18 elements (helium, neon, and argon. What Are Electron Shells Called.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology What Are Electron Shells Called an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or. What Are Electron Shells Called.
From mammothmemory.net
Electrons orbit the nucleus of an atom orbits are called she What Are Electron Shells Called the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. The energy levels for the electrons in an atom are often referred to as electron shells. electron shell,. What Are Electron Shells Called.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Are Electron Shells Called the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The energy levels for the electrons in an atom are often referred to as electron shells. energy levels (also called electron shells) are fixed distances. What Are Electron Shells Called.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free What Are Electron Shells Called energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. The energy levels for the. What Are Electron Shells Called.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Are Electron Shells Called group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. The energy levels for the electrons in an atom are often referred to as electron shells. A full. What Are Electron Shells Called.
From www.studocu.com
Electron Shell Levels Lecture Notes An electron shell is a set of What Are Electron Shells Called The energy levels for the electrons in an atom are often referred to as electron shells. the number of electron shells in an atom and the distribution of electrons in the shell determine the chemical. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. energy. What Are Electron Shells Called.
From www.bank2home.com
Electron Shells And Orbitals What Are Electron Shells Called A full valence shell is the most stable electron. energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The energy levels for the electrons in an atom are. What Are Electron Shells Called.
From cartoondealer.com
Periodic Table Of Element Showing Electron Shells Cartoon Vector What Are Electron Shells Called energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The energy levels for the. What Are Electron Shells Called.
From www.nagwa.com
Question Video Identifying the Electron Shells with the Most and the What Are Electron Shells Called The energy levels for the electrons in an atom are often referred to as electron shells. A full valence shell is the most stable electron. energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. group 18 elements (helium, neon, and argon are shown in figure 2) have. What Are Electron Shells Called.
From web.facebook.com
JayZ ARRESTED In Puerto Rico By Foreign POLICE After FLEEING FBI'S What Are Electron Shells Called A full valence shell is the most stable electron. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. The energy levels for the electrons in an atom are often referred to as electron shells. the number of electron shells in an atom and the distribution of. What Are Electron Shells Called.